
Dec 19, 2020 | Sci-Tech
Writes David Wallace-Wells in New York Magazine, "We Had the Vaccine the Whole Time":You may be surprised to learn that of the trio of long-awaited coronavirus vaccines, the most promising, Moderna’s mRNA-1273, which reported a 94.5 percent efficacy rate on November 16, had been designed by January 13. This was just two days after the genetic sequence had been made public in an act of scientific and humanitarian generosity that resulted in China’s Yong-Zhen Zhang’s being temporarily forced out of his lab. In Massachusetts, the Moderna vaccine design took all of one weekend. It was completed before China had even acknowledged that the disease could be transmitted from human to human, more than a week before the first confirmed coronavirus case in the United States. By the time the first American death was announced a month later, the vaccine had already been manufactured and shipped to the National Institutes of Health for the beginning of its Phase I clinical trial. This is — as the country and the world are rightly celebrating — the fastest timeline of development in the history of vaccines. It also means that for the entire span of the pandemic in this country, which has already killed more than 250,000 Americans, we had the tools we needed to prevent it .
The author then goes on to regurgitate "the FDA has to approve it" excuse for not banning the sale of the vaccine until the end of 2020:
To be clear, I don’t want to suggest that Moderna should have been allowed to roll out its vaccine in February or even in May, when interim results from its Phase I trial demonstrated its basic safety.
Well, why the hell not?
Shouldn't that judgment on the efficacy of the vaccine be up to each individual? If you have a high probability of dying from COVID-19 if you get it, the vaccine in February 2020 might be worth the risk.
And why not release it in May 2020 when it was proven "safe" by FDA standards (but not yet proven as "efficacious").
Observe that for 41% of voters; the pandemic was the "most important issue facing the country":

What was the FDA waiting for? For Trump to lose the 2020 Presidential election? I seriously hope not. More likely, it is something worse: bureaucratic inertia with a central planning anti-free-market mindset.
Continues the author on the "reasoning" of the experts:
An unsafe vaccine, like the one for polio that killed ten and paralyzed 200 in 1955, could cause medical disaster and public-health backlash — though, as Balloux points out, since none of the new coronavirus vaccines use real viral material, that kind of accident, which affected one in a thousand recipients, would be impossible. (These days, one adverse impact in a million is the rule-of-thumb threshold of acceptability.) An ineffective vaccine could also give false security to those receiving it, thereby helping spread the disease by providing population-scale license to irresponsible behavior (indoor parties, say, or masklessness). But on other matters of population-level guidance, our messaging about risk has been erratic all year, too. In February and March, we were warned against the use of masks, in part on the grounds that a false sense of security would lead to irresponsible behavior — on balance, perhaps the most consequential public-health mistake in the whole horrid pandemic. In April, with schools already shut, we closed playgrounds. In May, beaches — unable or unwilling to live with even the very-close-to-zero risk of socializing outside (often shaming those who gathered there anyway). But in September, we opened bars and restaurants and gyms, inviting pandemic spread even as we knew the seasonality of the disease would make everything much riskier in the fall. The whole time, we also knew that the Moderna vaccine was essentially safe. We were just waiting to know for sure that it worked, too.None of the scientists I spoke to for this story were at all surprised by either outcome — all said they expected the vaccines were safe and effective all along. Which has made a number of them wonder whether, in the future, at least, we might find a way to do things differently — without even thinking in terms of trade-offs.
The problem is that "scientists" cannot determine the "trade-offs" for any given individual. Those decisions should be up to the individual, with "experts" providing the facts, allowing each person to decide based on their particular situation and personal priorities, with the government's job to get-out-of-the-way.Given that the FDA blocked the sale and distribution of a vaccine that could have prevented the death of over a quarter a million Americans, we think the name suggested by Harry Binswanger to be a far more accurate description: Federal Death Agency.
Recommended Reading:
Dec 18, 2020 | Sci-Tech
 Note: In NY if a person contracted COVID-19 in LTC facility and dies in the hospital, NY counts it as a hospital death and does not attribute it the LTC.
Note: In NY if a person contracted COVID-19 in LTC facility and dies in the hospital, NY counts it as a hospital death and does not attribute it the LTC."...the Long-Term Care COVID Tracker is the most comprehensive dataset about COVID-19 in US long-term care facilities. It compiles crucial data about the effects of the pandemic on a population with extraordinary vulnerabilities to the virus due to age, underlying health conditions, or proximity to large outbreaks.The dataset compiles all currently available information of COVID-19 cases and related deaths in long-term care facilities—nursing homes, skilled nursing facilities, assisted living facilities, and other care homes—and tracks both residents and staff.
One solution is to "bubble" the home and have staff live full-time on-site during the pandemic:Currently, most senior homes rely on checkpoints to screen staff as they arrive to work, mainly by asking them questions and taking their temperatures. But these checkpoints can easily fail, because people without symptoms can carry and transmit the coronavirus. Moreover, many staff members work at multiple homes or have family members who work at other facilities. Many senior homes also have been preparing for the pandemic by hiring extra staffers. So it is hardly surprising that the contagion has spread like a chain reaction in senior care homes.[...]A better approach is to pay front line aides and nurses to live on-site through the period when the disease is surging — meaning right now. This is hardship work, requiring staff to work 60 to 80 hours a week without seeing family members. But it could be the best way to protect our elderly. Lowering the number of infections at our senior homes would also allow us to conserve protective equipment, reduce the need for hospital beds and prevent the spread of the disease into communities where staff members live.[...]At homes overwhelmed by Covid-19, having caregivers live on-site would prevent them from bringing the virus home to their families or spreading it through communities, particularly when they commute.
Looking ahead, Covid may recede for much of the country this summer, but I fear that senior homes will remain vulnerable to a new wave of infection. We can prepare for that by having our staff live in our homes.
The result?The result has been promising; we have yet to have a confirmed case of Covid-19 among our residents or staff.
Unfortunately, it is more expensive:But I cannot afford it for much longer, and many other senior care centers could not afford to even start such a program.
Hat Tip: Phil Magness



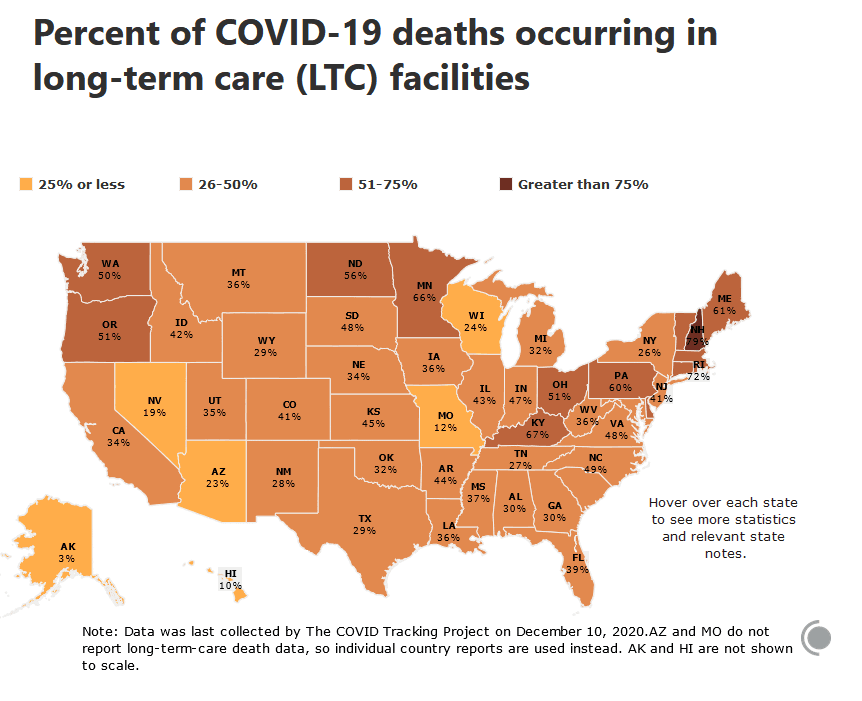 Note: In NY if a person contracted COVID-19 in LTC facility and dies in the hospital, NY counts it as a hospital death and does not attribute it the LTC.
Note: In NY if a person contracted COVID-19 in LTC facility and dies in the hospital, NY counts it as a hospital death and does not attribute it the LTC.